
Fats are one of the major groups of biological molecules made up from carbon, oxygen, and hydrogen. They differ from carbohydrates (made from the same elements) in that the hydrogen and oxygen are not in the same proportions as in water. The first typical characteristic of fats is that they are hydrophobic (water-fearing) and therefore they do not dissolve in water, but they form small droplets dispersed in water, or they flow on the top of water. Chemical analysis of fats shows the large proportion of hydrogen present in them. For example, trierucin (the main component of rape seed oil) has a composition of C69H128O6. This points to their second typical characteristic – the strongest relation to the element of warmth, shown in the amount of dietary calories they provide (9 kcal in comparison to about 4 kcal provided by carbohydrates and proteins).
Lipids is the modern scientific designation of fats. The scientific rationale is that "although the words 'oils', 'fats', and 'lipids' are all used to refer to fats, in reality, fat is a subset of lipid." The spiritual-scientific rationale is that behind fats there are active specific spiritual forces (see THE TRUE NATURE OF NUTRITIVE SUBSTANCES) and this is the primary cause for their specific characteristics. For that reason one can also count as subsets of fats all the groups of lipids listed in scientific classification.
Triglycerides is another scientific designation of fats based on their core structure composed from one unit of glycerol and three of fatty acids. Glycerol (C3H8O3) is a central molecule that serves as the backbone to which fatty acids are linked.
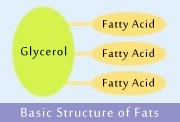
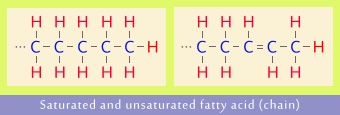
Fatty acids that are combined with glycerol have long carbon chains, with as many as 18 carbon atoms. Saturated fatty acid chain is full up (saturated) with hydrogen atoms. Unsaturated fatty acid chain contains one or more double bonds between carbon atoms, and consequently is not saturated with hydrogen atoms to the full extent.
Unsaturated fatty acids are further divided into:
Among them are two fatty acids which are spoken about frequently in the field of nutritional research:
Trans fatty acids are a group of fatty acids occurring naturally in small amounts (2 to 5%) in milk and meat and in large amounts (up to 45%) in foods made with partially hydrogenated oils. This process involves heating the unsaturated oils under pressure with hydrogen and a metal catalyst, causing them to become solid at room temperature. The additional 'benefit' of these industrially made fats is that they are more resistant to become rancid – meaning a much longer shelf life. Thus we have to distinguish natural from artificial trans fatty acids (also called trans fats).