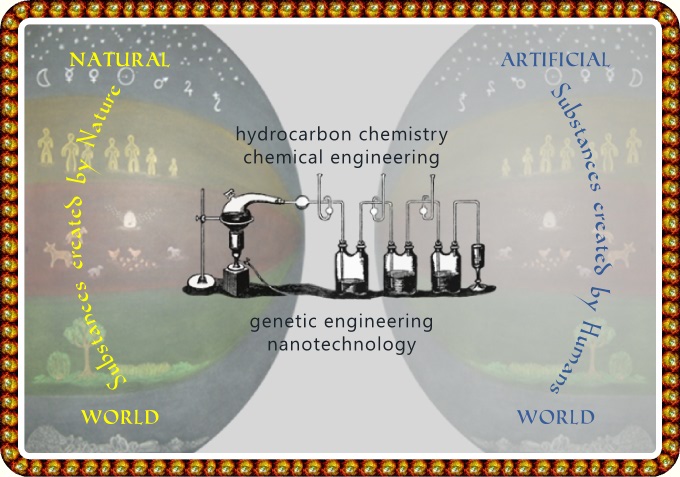
The Origin and Character of Natural vs Artificial Substances
We can introduce this topic with the common sense definition of “natural
compounds referring to those that are produced by plants or animals” [1] –
that is, substances produced by living organisms. And we could define
artificial substances as those which are produced in laboratories by means
of chemical or genetic engineering. But things are not so simple. For in
nature we also find minerals, such as stones, rocks and whole mountain
ranges which are not produced by plants or animals. And then we have coal,
crude oil and gas which originate from the remnants of living organisms from
the past geological epochs. Besides, throughout millennia human beings have
been using biological processes which cause chemical changes in substances.
What is the difference between these methods and modern chemical engineering?
In fact, the greatest amount of chemical transformation is happening in
nature itself. Plants are chemical geniuses in
natural chemistry in comparison with animals and
human beings. They are capable of producing not just living organic
substances from minerals, water, carbon dioxide and light, but an enormous
array of chemical compounds
of all sorts. Only in the last two decades have scientists tapped into this
realm of so-called phytochemicals, and started to figure
out their chemical structures (formulas) and give them names (e.g.
indole-3-carbinol, 16-alpha-hydroxyestrone, dihydroacetoxymatricin, etc). As an example you can look at
The Chemistry Found in a Single Yarrow Plant to
see for yourself the amazing power of ‘chemical engineering’ performed by the
plant kingdom. This is not surprising when by means of spiritual science we
get to know the twofold nature of plants. Because plants contain the physical and the
etheric body we can see them as true representatives of the
power of etheric body. This body is composed of four ethers – among them the
chemical ether.
The presence of this ether which governs chemical processes
and its kinship with the element water explains the extraordinary ability of
plants to handle chemical processes. [2]
In comparison with this the essential character of chemical
engineering is the human ability to manipulate
chemical reactions
with the aim to produce specific substances we require. For that purpose
we use two basic groups of chemical reactions:
- Reactions where two or more substances combine to
form new substance. The general formula is: A + B = AB
- Reactions where one substance breaks down into its constituent parts.
The general formula is: AB = A + B
In modern chemistry this twofold division is somehow obscured by an
extended list of chemical reactions which are no more than variations of
these two basic processes of combining and dividing. However, with little
effort we can see the existence of two groups of chemical processes. In one
group are chemical processes of synthesis,
and in another processes of decomposition. [3]
Although in the previous centuries chemists had learned to make
several chemical substances in a laboratory conditions, the great
breakthrough came in the second half of the nineteenth century, in a
period of so-called ‘second industrial revolution’ – a revolution based on gas, oil, electricity and
chemical engineering. This was the time of
the emergence of coal tar chemistry,
petroleum chemistry and
pharmaceutical industry. This was the time when chemists discovered how to make
the first synthetic dyes, perfumes, and sweeteners; they learned to make
the first artificial rubbers, adhesives,
fertilisers,
pesticides,
plastics and drugs. There was a further development of their skills in
the twentieth century by taking substances derived from plant or animal
sources and transforming them by means of chemical engineering.
[4] This was also the period of emergence and great
expansion of food additives. In fact, only since the middle of the
nineteenth century has there existed the need to distinguish between
natural and artificial substances in our food chain; in previous times
such differentiation would be senseless and superfluous.
Chemical engineering works on the level of
inorganic substances –
that is, the chemical elements
arranged in the periodic table.
Here we are dealing with single elements (atoms) or molecules
(chemical compounds). These substances belong to the inorganic mineral
kingdom. In the living kingdoms of nature – including plants, animals,
human beings, and the extraordinarily realm of
microorganisms
[5] – we have further structural layers: cells,
tissues, and organs. The entrance of scientists into these realms of
living organisms with the same methods of manipulation can be seen as a
kind of ‘natural development’. However, this has brought into existence
the controversial methods of manipulation of the genetic codes of cells
and microorganisms. This is the origin of genetic engineering
in a laboratory environment where people by their choice try to transfer
a specific characteristic that is not typical for the organism in
question. The latest method of manipulation is nanotechnology
by which the matter is manipulated on an atomic, molecular, and
supramolecular scale of existence. [6]
What all these methods have in common is that they are performed by
human beings in a laboratory environment – either research or factory
laboratories – and that in this manner a change occurs inside the three
lowest structural levels of substances: atomic, molecular and cellular
levels. These changes can be in the chemical structure of substance or
in the genetic structure of a cell. Thus we have arrived at the key
character of artificial substances.

On the basis of this we can characterize natural substances as:
[7]
- Substances which has been produced ‘in natura’. An
exception from this are hydrocarbons.
- These substances can be either inorganic or organic. [8]
- They can be physically extracted, but not altered in their inner structure.
- They can be altered by means of naturally occurring biological
processes, such as lactic or alcoholic fermentation, etc.
With the help of this ‘definition’ the following conclusions are evident:
- ‘Artificial’ is not a synonym for ‘synthetic’;
although all synthetic substances are artificial, we have beside them other
artificial substances which are not created by means of chemical
engineering. These are genetically modified organisms, those produced by
nanotechnology, etc.
- It is irrelevant if people aim by means of chemical engineering to
simulate natural processes, or if they simply wish to create a new
substance. So called ‘nature-identical’ substances and synthetic substances
are both artificial substances, for they are both produced in laboratory
environment.
- There is a distinction between mineral substances refined from plant
material (e.g. sugar) and mineral substances produced by chemical processes
(e.g.
sucralose). In the first case we cannot call them artificial, because in
its production only physical methods of extraction are used, while in the
second case chemical engineering is used to obtain what is required. [9]
The Reverse Chemistry of Subnatural Hydrocarbons
Among the substances found in the earth’s core are also substances which
cannot be counted as natural. While ores are counted as natural substances
until they are subjected to the processes of chemical engineering,
substances like coal, crude oil, natural gas, and similar substances, are in
their basis artificial. Behind this seeming paradox is the hidden reason
which provides a very good argument in favour of this exception.
These substances have been produced from the remnants of ancient plant
and animal organisms by ‘geological chemistry’ of the earth, under extreme
pressures and temperatures. These processes have caused the profound changes
in their chemical compositions. The most important change is the complete
loss of oxygen. Their chemical structures have similar carbon frameworks
like carbohydrates, but without any oxygen. For that reason they are called
hydrocarbons. From a
spiritual-scientific perspective they are dead substances, because they lack
the element of oxygen, the carrier of cosmic life in nature.
[10] The realm where these substances can be found lies below the visible
kingdoms of nature and is therefore called ‘subnature’. For that reason
these substances are not regarded as natural; however, they could be called
‘subnatural’.
It is an interesting historical fact that the emergence of modern
chemical engineering is linked with the emergence of
coal tar chemistry.
Coal was the main source of energy in the first phase of the industrial
revolution. Amongst other uses it was utilized for the production of
coke and illuminating gas.
“A by-product of coke and gas industry was
coal tar. At first it was just a nuisance, because of its resistance to
chemical and oxidizing agents. The main breakthrough happened with the
discovery that a combination of sulphuric and nitric acids could break down the
cycloparaffins,” [11]
the main chemical compounds of coal tar.
With further chemical discoveries coal tar and later petroleum have
become the mainstay of the modern chemical industry.
Now if we compare the natural chemistry of plants with the outcomes
of the coal tar and petroleum chemistry we can see the following:
[12]
- Plants are capable of producing not just living
substances such as starches, fats, proteins, but also sugars, including
nectar. Besides this plants produce numerous chemical compounds which create
colours, scents, etheric oils, and healing substances. Today we call these substances
phytochemicals.
- Human beings have succeeded in producing from hydrocarbons artificial
copies of natural substances. In the laboratory environment “human ingenuity
takes hold and conjures forth a synthetic mirror-image of the natural world:
synthetic colours, scents,
saccharine and
other sweeteners, mineral oils and synthetic medicaments.” [13]

“Contrasting the two realms, we get the impression that the upper
one is the realm of dynamic biological reality, the scene of a ceaseless
harmonization of the living polarities of earth and heaven, giving rise
to an endless range of metamorphoses. The underworld of the chemistry of
hydrocarbons, on the other hand, seems – figuratively speaking – like
ghostly reflections of the dynamic creativity of the cosmos. Despite the
calculable certainties found in this sub-earthly realm, it cannot seem
more real to us than that of the greening, flowering and fruiting
plants.” [14]
Why was it possible to create from hydrocarbons the spectrum of
artificial substances which are as a mirror-image of natural substances?
This is due to the fact, that the primary origin of hydrocarbons is the
antediluvian
world of plants and lower animals – all these substance once upon the
time belonged to the living natural world. But in the meantime they have
gone twice through the process of change of their chemical structure:
- First time in the ‘geological laboratory of
subnature’ where they were transformed into coal, lignite,
petroleum, gas and similar.
- Second time in the human laboratories and chemical factories where
they were ‘reversed back’ into artificial imitations of natural substances.
For that reason we could define them as double-artificial substances, or
‘artificial on square’. The hydrocarbons are a good source of energy for
transport and industry, but for the sake of human wellbeing they should
have never entered the food chain in any form.